NON-HODGKIN
LYMPHOMA (NHL)
Exploring dysfunctional pathways, mechanisms, and biomarkers in non-
Hodgkin lymphoma to discover new insights into the progression of the
disease.
Relevant Cancer Targets
- Fitzmaurice, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553-1568.
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
- American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society; 2018.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Global Burden of Disease Cancer Collaboration; Sung H; Ferlay J, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J. Clin. 2021;71(3):209-249.
- Vose JM. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2017;92(8):806-813.
- Leukemia & Lymphoma Society. NHL Subtypes. http://www.lls.org/lymphoma/non-hodgkin-lymphoma/diagnosis/nhl-subtypes. Accessed November 2020.
- Glass S, Phan A, Williams JN, Flowers CR, Koff JL. Integrating understanding of epidemiology and genomics in B-cell non-Hodgkin lymphoma as a pathway to novel management strategies. Discov Med. 2016;21(115):181-188.
- Mayo Foundation for Medical Education and Research. Diseases and Conditions: Non-Hodgkin's Lymphoma: Risk Factors. http://www.mayoclinic.org/diseases-conditions/non-hodgkins-lymphoma/basics/risk-factors/con-20027792. Accessed November 2020.
- Mahmoud HM, El-Sakhawy YN. Significance of Bcl-2 and Bcl-6 immunostaining in B-non Hodgkin's lymphoma. Hematol Rep. 2011;3(3):e26.
- Wang J, Zhou M, Xu J, Chen B, Ouyang J. Combination of BCL-2 and MYC protein expression improves high-risk stratification in diffuse large B-cell lymphoma. Onco Targets Ther. 2015;8:2645-2650.
- Scott DW, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848-2856.
- Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2003;101(6):2125-2131.
- Parekh S, Weniger MA, Wiestner A. New molecular targets in mantle cell lymphoma. Semin Cancer Biol. 2011;21(5):335-346.
- National Cancer Institute. Adult Non-Hodgkin Lymphoma Treatment (PDQ®)-Health Professional Version. https://www.cancer.gov/types/lymphoma/hp/adult-nhl-treatment-pdq. Accessed November 2020.
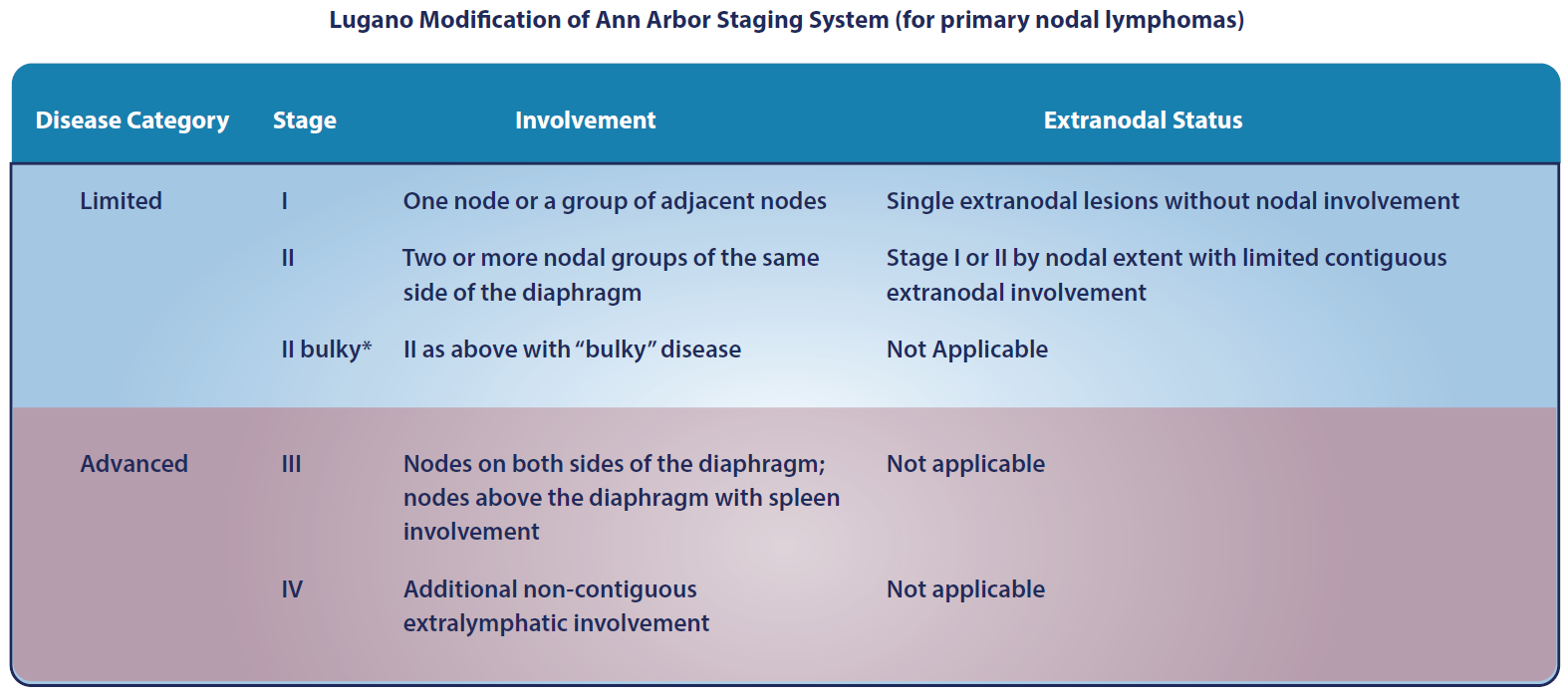
- Cheson B, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3067.
- Amin AD, Peters TL, Li L, et al. Diffuse large B-cell lymphoma: can genomics improve treatment options for a curable cancer? Cold Spring Harb Mol Case Stud. 2017;3(3):a001719.
- Press OW, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus 131iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol. 2013;31(3):314-320.
- Casulo C, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516-2522.
- Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127(23):2804-2808.
- Kahl B. Is there a role for "watch and wait" in follicular lymphoma in the rituximab era? Hematology Am Soc Hematol Educ Program. 2012;2012:433-438.
- Fakhri B, Kahl B. Current and emerging treatment options for mantle cell lymphoma. Ther Adv Hematol. 2017;8(8):223-234.
- Parrott M, Rule S, Kelleher M, Wilson J. A systematic review of treatments of relapsed/refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2017 Oct 13. [Epub ahead of print]
- Smith SM, et al. Aggressive B-cell lymphoma: the double-hit and double-expressor phenotypes. Clin Adv Hematol Oncol. 2017;15(1):40-42.
- Barton S, et al. Are we ready to stratify treatment for diffuse large B-cell lymphoma using molecular hallmarks? Oncologist. 2012;17(12):1562-73.
- Riedell PA, et al. How to Refine Treatment Choice in Follicular Lymphoma: From Low-Tumor Burden to High-Risk Follicular Lymphoma. Am J Hem/Onc. 2016;12(6):15-19.
- Hoster E, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-65.
- Sarkozy C, et al. Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer. 2014;53(1):106-16.
- Olszewski AJ, et al. Survival of patients with marginal zone lymphoma. Cancer. 2013 Feb 1;119(3):629-38.
- Lai R, et al. Frequency of bcl-2 expression in non-Hodgkin's lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol. 1998 Sep;11(9):864-869.
- Castillo JJ, et al. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: an analysis of the Surveillance, Epidemiology and End Results database. Br J Haematol. 2015;169(1):81-9.
- Kyle RA, et al. Prognostic markers and criteria to initiate therapy in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003 Apr;30(2):116-20.
- Treon SP, et al. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123(18):2791-2796.
- Castillo JJ, et al. Future therapeutic options for patients with Waldenström macroglobulinemia. Best Practice & Research Clinical Haematology 29 (2016) 206-215.
- Zucca E, et al. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127(17):2082-2092.
- Thieblemont C, et al. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127(17):2064-2071.
- Arcaini L, et al. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072-2081.
- Rummel MJ, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-10.
- Kastritis E, et al. Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenström macroglobulinemia: final analysis of a phase 2 study. Blood 2015. 126(11):1392-4.
- Dimopoulos MA, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122(19):3276-82.
- Klasa RJ. Targeting the proapoptotic factor Bcl-2 in non-Hodgkin's lymphoma Oncology. (Williston Park). 2004 Nov;18(13 Suppl 10):25-31.
- NCI. Cancer Stat Facts: Non-Hodgkin Lymphoma (NHL). https://seer.cancer.gov/statfacts/html/nhl.html. Accessed September 2021.