BCL-2
OVERVIEW
B-cell lymphoma 2 (BCL-2) is an anti-apoptotic, pro-survival member of the BCL-2 family of proteins that
function to regulate the intrinsic pathway of apoptosis, or programmed cell death, and help maintain cellular
homeostasis.1,2
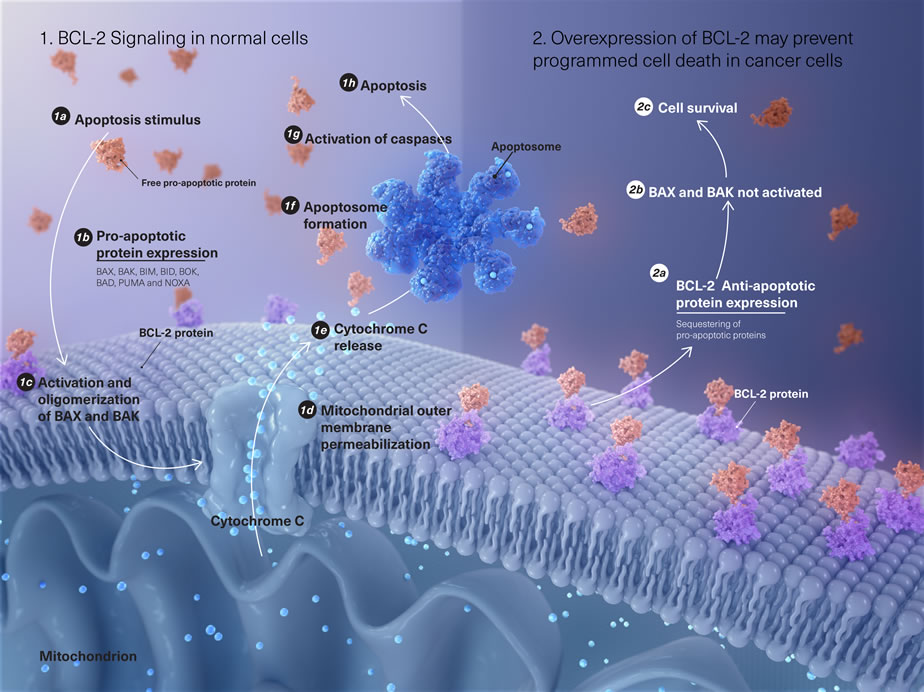
BCL-2 family proteins are key regulators of the intrinsic apoptotic pathway and include both pro-survival (anti-apoptotic) and pro-death (pro-apoptotic) proteins with opposing functions. Apoptosis is regulated by the balance of these BCL-2 family proteins.1
- Anti-apoptotic proteins: BCL-2, BCL-XL, BFL-1/A1, BCL-W and MCL-12-4
- Pro-apoptotic multi-domain effector proteins: BAX, BAK, BOK2-4
- Pro-apoptotic BH3-only proteins: BIM, BID, BAD, PUMA and NOXA2-4
The BH3-only proteins are further subdivided into two groups based on function: the "activators" (such as BIM and PUMA) and the "sensitizers" (such as NOXA and BAD).4,6
The BCL-2 family of proteins controls cell death primarily by direct binding interactions that regulate mitochondrial outer membrane permeabilization (MOMP), a process leading to the irreversible release of intermembrane space proteins, subsequent caspase activation and apoptosis.7
- The affinities and relative abundance of the BCL-2 family proteins dictate interactions between anti-apoptotic and pro-apoptotic BCL-2 family proteins that regulate MOMP.7
IMPLICATIONS IN CANCER
Cytotoxic stresses (e.g. DNA damage or oxidative stress) may activate pro-death BH3-only proteins, which can initiate apoptosis in two ways:
- The "activators" are often bound and sequestered by pro-survival proteins. When unbound, they can directly interact with pro-death "effectors" BAX and BAK, leading to oligomerization and insertion into the mitochondrial outer membrane. BAX and BAK then assemble to form pores, leading to mitochondrial outer membrane permeabilization (MOMP), the release of cytochrome c into the cytosol, the formation of the apoptosome, and caspase activation, which eventually result in apoptosis2,6,8
- The "sensitizers" can only bind to the pro-survival proteins, which neutralizes them and allows for the release of the "activator" and "effector" proteins to drive the initiation of apoptosis2,6,8,9
Malignant cells often evade apoptosis by upregulating BCL-2 and other pro-survival proteins (such as MCL-1 and BCL-XL).3,4,6,8,9
Some malignant cells lie close to the apoptotic threshold but are held back from death by the pro-survival BCL-2 family proteins. In such cells, the increased expression of these pro-survival proteins (BCL-2, MCL-1, and/or BCL-XL) allows them to sequester and inactivate pro-death proteins, thereby resisting cytotoxic stress-induced apoptosis.3,4,6,8,9
- Pro-survival proteins have been shown to be expressed at high levels in many hematologic malignancies, where they can sequester pro-death proteins, resulting in malignant cell survival.3,4,6,8,9
Cells with a large pool of bound and inactivated pro-death proteins are said to be "primed for death," meaning they are on the verge of freeing sufficient pro-death proteins to initiate apoptosis.3,4,9
Agents that inhibit pro-survival proteins (e.g., BCL-2, BCL-XL, or MCL-1 inhibitors) can also promote apoptosis in malignant cells.3-5, 10
ONCOGENIC EXPRESSION
The majority of tumors have defects in the p53 pathway and many overexpress BCL-2 or a close relative, such as BCL-XL.2
- Most CLLs, FLs, and MCLs were found to have moderate to high expression of BCL-2 and BCL-XL.11
- BCL-2, frequently overexpressed in follicular lymphomas bearing the t(14;18) chromosomal translocation, is also widely expressed in myeloma cell lines.12
As a pro-survival oncoprotein which can be overexpressed as a result of cancer-specific mutations or gene amplifications, BCL-2 clearly deserves consideration for therapeutic inhibition.13
Acute Myeloid Leukemia (AML) / Myelodysplastic Syndromes (MDS)
- The BCL-2 protein is overexpressed in up to 70% of AML cases.16-17
- Elevated levels of BCL-2 correlate with poor prognosis and chemoresistance.18-21
- A high number of BCL-2-addicted cell lines and patient samples are sensitive to selective BCL-2 inhibition.22
- Sensitivity to BCL-2 inhibition can be promoted by directly or indirectly downregulating or neutralizing other pro-survival proteins (e.g., with proteasome inhibitors, hypomethylating agents, or LDAC).4
Non-Hodgkin Lymphoma (NHL)
Mantle Cell Lymphoma (MCL)
- The BCL-2 pathway is commonly deregulated in MCL cases and the BCL2 gene is often amplified.23,24
- The BCL-2 protein is overexpressed in up to 97% of MCL cases.11,25
- Chromosome 18q21 amplification leading to high BCL-2 protein levels has been reported in a subset of patients with MCL.25,26
Follicular Lymphoma (FL)
- BCL-2 protein overexpression is detected in over 60% of FL cases.18
- Approximately 85% of patients with FL have t(14;18), a translocation between chromosomes 14 (Ig) and 18 (BCL2). Of those FL patients with t(14;18) translocation, up to 90% overexpress BCL-2 protein.28-30
- The vast majority of patients with BCL-2 protein overexpression develop chemoresistance and experience higher relapse rates compared with BCL-2-negative/normal patients.31
- Mutations in the BCL-2 coding sequence are associated with shorter times to transformation to a more aggressive lymphoma and earlier death.32
Diffuse Large B-Cell Lymphoma (DLBCL)
- BCL-2 protein overexpression is detected in up to 40% of DLBCL cases.18,33
- DLBCL may also be classified as "double-hit" lymphoma or "double-expression" lymphoma based on MYC and BCL2 gene characteristics.34,35
- "Double expression", where MYC and BCL-2 are overexpressed at the protein level, may be present in as many as one-third of patients with DLBCL and correlates with an aggressive clinical course and a poor outcome.33,34
- "Double hit", the presence of both the MYC and BCL2 rearrangements, occurs in approximately 5% to 7% of patients with DLBCL and is associated with highly proliferative and drug-resistant disease leading to a poor prognosis.34
Multiple Myeloma (MM)
- Multiple myeloma is a heterogenous disease, and the rate of progression may be influenced by underlying cytogenetic type of disease. Primary cytogenetic abnormalities like translocations involving the IgH locus can influence disease course, response to therapy, and prognosis. IgH translocations such as t(11;14), t(4;14), t(6;14), t(14;16), and t(14;20) are identified in approximately 40% of MM patients. The most common IgH translocation in MM is t(11;14) and is present in approximately 20% of patients.36
- Multiple myeloma harboring t(11;14) represents a unique disease subset as t(11;14)-positive myeloma cells exhibit biological features that are distinct from t(11;14)-negative myeloma cells.37
- t(11;14) MM cells often lack traditional plasma cell markers that are detected on other MM cell types and exhibit remnants of B-cell biology. Expression of the B-cell lineage membrane protein CD20 and higher levels of the B-cell receptor component CD79a have been detected in t(11;14) MM cells.
- MM cells carrying t(11;14) have been shown to express a higher degree of anti-apoptotic BCL-2 relative to MCL-1. Both normal plasma cells and most MM cells without t(11;14) primarily depend on the anti-apoptotic protein MCL-1 for survival.
- t(11;14) MM cells have been confirmed to retain features of B-cell biology, including lower mitochondrial metabolism and evidenced by significantly lower Mito signature scores detected in t(11;14)-positive versus t(11;14)-negative R/R MM patients39
- Further, preclinical data shows that the combination of dexamethasone and venetoclax enhances the dependency on BCL-2 for survival.38
- Co-treatment of human myeloma cell lines and primary patient samples with dexamethasone and venetoclax significantly increased cell death over venetoclax alone.
- The mechanism by which this occurs is an increase in the expression of both BCL-2 and BIM upon addition of dexamethasone which shifts BIM binding towards BCL-2 resulting in increased sensitivity to venetoclax.
Related Research
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590-8607.
- Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb). 2011;3:279-296.
- Czabotar PE, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014; 15:49–63.
- Leverson JD, et al. Found in Translation: How Preclinical Research Is Guiding the Clinical Development of the BCL2-Selective Inhibitor Venetoclax. Cancer Discov. 2017; 7:1376–1393.
- Valentin R, et al. The rise of apoptosis: targeting apoptosis in hematologic malignancies. Blood. 2018; 132:1248–1264.
- Letai A, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002; 2:183–192.
- Kale J, et al. BCL-2 family proteins: changing partners in the dance towards death. Cell Death and Differentiation. 2018;25:65–80.
- Adams JM & Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007; 26:1324–1337.
- Certo M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006; 9:351–365.
- Souers AJ, et al. Nat Med 2013; 19:202–208 (incl. suppl.)
- Agarwal B, et al. Bcl-2 family of proteins in indolent B-cell non-Hodgkin's lymphoma: study of 116 cases. Am J Hematol. 2002 Aug;70(4):278-82.
- Trudel S, Stewart AK, Li Z, et al. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clin Cancer Res. 2007;13(2):621-629.
- Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30(25):3127-3135.
- Anderson MA, Huang D, Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol. 2014;51(3):219-227.
- Hanada M, et al. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993;82(6):1820-1828.
- Campos L, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091-3096.
- Mehta SV, et al. Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: its correlation with FLT3. Neoplasma. 2013;60(6):666-675.
- Mahmoud HM, et al. Significance of Bcl-2 and Bcl-6 immunostaining in B-non Hodgkin's lymphoma. Hematol Rep. 2011;3(3):e26.
- Parker JE, et al. The role of apoptosis, proliferation, and the Bcl–2–related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000;96(12):3932-3938.
- Schimmer AD. Novel therapies targeting the apoptosis pathway for the treatment of acute myeloid leukemia. Curr Treat Options Oncol. 2007;8(4):277-286.
- Del Poeta G, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood. 2002;101(6):2125-2131.
- Pan R, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362-375.
- Perez-Galan P, et al. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26-38.
- Parekh S, et al. New molecular targets in mantle cell lymphoma. Semin Cancer Biol. 2011;21(5):335-346.
- Tracey L, et al. Expression of the NF-nB targets BCL2 and BIRC5/survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. JPathol. 2005;206:123–134.
- Davids MS. Targeting BCL-2 in B-cell lymphomas. Blood. 2017;130(9):1081-1088.
- Bentz M, et al. t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2000;27(3):285-294.
- Freedman A. Follicular lymphoma: 2018 update on diagnosis and management. Am J Hematol. 2018;93(2):296-305.
- Vaandrager et al. Interphase FISH detection of BCL2 rearrangement in follicular lymphoma using breakpoint-flanking probes. Genes Chromosomes Cancer. 2000, 27:85-94.
- Skinnider BF. et al. Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Human Pathology. 1999. 30(7):803-808.
- Kang MH, Reynolds CP. Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clinical Cancer Research. 2009;15(4):1126-1132.
- Correia C, et al. BCL2 mutations are associated with increased risk of transformation and shortened survival in follicular lymphoma. Blood. 2015;125(4):658-667.
- Wang J, et al. Combination of BCL-2 and MYC protein expression improves high-risk stratification in diffuse large B-cell lymphoma. Onco Targets Ther. 2015;8:2645-2650.
- Smith SM, et al. Aggressive B-cell lymphoma: the double-hit and double-expressor phenotypes. Clin Adv Hematol Oncol. 2017;15(1):40-42.
- Punnoose EA, et al. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol Cancer Ther. 2016;15(5):1132-1144.
- Rajkumar SV, et al. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:1086-1107.
- Bal S, et al. Multiple myeloma with t (11; 14): unique biology and evolving landscape. Am. J. Cancer Res. 2022;12(7):2950.
- Matulis SM, et al. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016;30(5):1086-93.
- Sharon D, et al. Clinical Genomic Analyses Demonstrate t(11;14) Multiple Myeloma Retains B-cell Biology and Distinct Mitochondrial Metabolism That Convey Increased Sensitivity to BCL-2 Inhibition by Venetoclax. Poster #. 64th Annual ASH Meeting and Exposition. December 10-13, 2022. New Orleans, LA.