NON-HODGKIN
LYMPHOMA (NHL)
Exploring dysfunctional pathways, mechanisms, and biomarkers in non-
Hodgkin lymphoma to discover new insights into the progression of the
disease.
- Fitzmaurice, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553-1568.
- Howlader N, Noone AM, Krapcho M, et al (eds). National Cancer Institute. SEER Cancer Statistics Review, 1975-2017. Updated April 2020 based on November 2019 SEER data submission. https://seer.cancer.gov/csr/1975_2017/.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- International Agency for Research on Cancer. (2022). GLOBOCAN: Global Cancer Observatory. World Health Organization. https://gco.iarc.who.int/
- Blood Cancer United. 2025. https://bloodcancerunited.org/blood-cancer/lymphoma/non-hodgkin-lymphoma-nhl. Accessed November 2025.
- Glass S, Phan A, Williams JN, Flowers CR, Koff JL. Integrating understanding of epidemiology and genomics in B-cell non-Hodgkin lymphoma as a pathway to novel management strategies. Discov Med. 2016;21(115):181-188.
- Mayo Foundation for Medical Education and Research. Diseases and Conditions: Non-Hodgkin's Lymphoma: Risk Factors. Accessed November 2025 http://www.mayoclinic.org/diseases-conditions/non-hodgkins-lymphoma/basics/risk-factors/con-20027792.
- Mahmoud HM, El-Sakhawy YN. Significance of Bcl-2 and Bcl-6 immunostaining in B-non Hodgkin's lymphoma. Hematol Rep. 2011;3(3):e26.
- Wang J, Zhou M, Xu J, Chen B, Ouyang J. Combination of BCL-2 and MYC protein expression improves high-risk stratification in diffuse large B-cell lymphoma. Onco Targets Ther. 2015;8:2645-2650.
- Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2003;101(6):2125-2131.
- Parekh S, Weniger MA, Wiestner A. New molecular targets in mantle cell lymphoma. Semin Cancer Biol. 2011;21(5):335-346.
- National Cancer Institute. Adult Non-Hodgkin Lymphoma Treatment (PDQ®)-Health Professional Version. Accessed November 2025.https://www.cancer.gov/types/lymphoma/hp/adult-nhl-treatment-pdq.
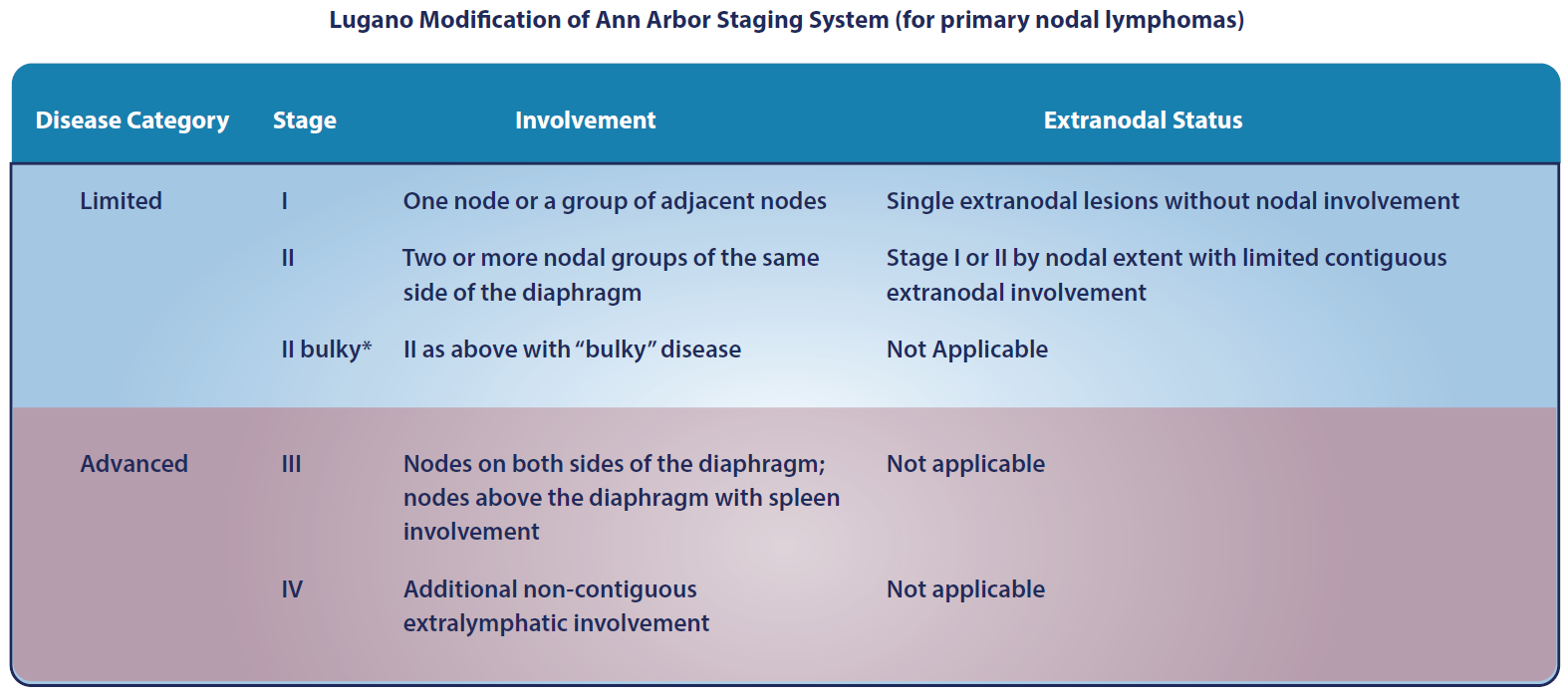
- Cheson B, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3067.
- Amin AD, Peters TL, Li L, et al. Diffuse large B-cell lymphoma: can genomics improve treatment options for a curable cancer? Cold Spring Harb Mol Case Stud. 2017;3(3):a001719.
- Press OW, et al. Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus 131iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016. J Clin Oncol. 2013;31(3):314-320.
- Casulo C, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33(23):2516-2522.
- Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127(23):2804-2808.
- Kahl B. Is there a role for "watch and wait" in follicular lymphoma in the rituximab era? Hematology Am Soc Hematol Educ Program. 2012;2012:433-438.
- Fakhri B, Kahl B. Current and emerging treatment options for mantle cell lymphoma. Ther Adv Hematol. 2017;8(8):223-234.
- Parrott M, Rule S, Kelleher M, Wilson J. A systematic review of treatments of relapsed/refractory mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):13-25.e6.
- Smith SM, et al. Aggressive B-cell lymphoma: the double-hit and double-expressor phenotypes. Clin Adv Hematol Oncol. 2017;15(1):40-42.
- Barton S, et al. Are we ready to stratify treatment for diffuse large B-cell lymphoma using molecular hallmarks? Oncologist. 2012;17(12):1562-73.
- Riedell PA, et al. How to Refine Treatment Choice in Follicular Lymphoma: From Low-Tumor Burden to High-Risk Follicular Lymphoma. Am J Hem/Onc. 2016;12(6):15-19.
- Hoster E, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-65.
- Sarkozy C, et al. Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer. 2014;53(1):106-16.
- Olszewski AJ, et al. Survival of patients with marginal zone lymphoma. Cancer. 2013 ;119(3):629-38.
- Lai R, et al. Frequency of bcl-2 expression in non-Hodgkin's lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol. 1998 ;11(9):864-869.
- Castillo JJ, et al. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: an analysis of the Surveillance, Epidemiology and End Results database. Br J Haematol. 2015;169(1):81-9.
- Kyle RA, et al. Prognostic markers and criteria to initiate therapy in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30(2):116-20
- Treon SP, et al. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123(18):2791-2796.
- Castillo JJ, et al. Future therapeutic options for patients with Waldenström macroglobulinemia. Best Pract Res Clinl Haematol. 2016;29(2):206-215.
- Zucca E, et al. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127(17):2082-2092.
- Thieblemont C, et al. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127(17):2064-2071.
- Arcaini L, et al. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072-2081.
- Rummel MJ, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-10.
- Kastritis E, et al. Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenström macroglobulinemia: final analysis of a phase 2 study. Blood 2015. 126(11):1392-4.
- Dimopoulos MA, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122(19):3276-82.
- Klasa RJ. Targeting the proapoptotic factor Bcl-2 in non-Hodgkin's lymphoma. Oncology. (Williston Park). 2004;18(13 Suppl 10):25-31.
- NCI. Cancer Stat Facts: Non-Hodgkin Lymphoma (NHL). Accessed March 2025. https://seer.cancer.gov/statfacts/html/nhl.html.
- Furtado M and Rule S. Indolent mantle cell lymphoma. Haematologica. 2011 Aug; 96(8): 1086–1088.